Abstract
Patients with hematological malignancies (HM) have an increased risk of severe coronavirus disease 2019 (COVID-19) and subsequent mortality. Moreover, patients with HM have prolonged viral shedding and long-term infectivity. However, the infectious period varies depending on the degree of immunosuppression caused by the disease itself and intensity of chemotherapy. The infectivity of the virus can be evaluated by the cycle threshold (Ct) value obtained from reverse transcription quantitative polymerase chain reaction (RT-qPCR), which is inversely proportional to the viral load. Viral cultures are less likely to be positive when Ct values exceed 30-35, which suggests that patients with Ct values above the threshold may be less infectious. Our study evaluated the trajectories of serial Ct values of patients with HM indicated for the test-based strategy and examined the relationship between patients' characteristics and the duration of viral shedding.
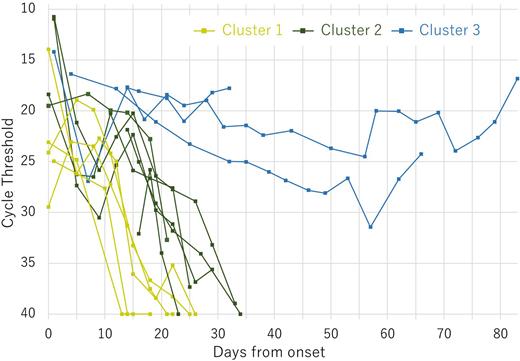
Seventeen patients with HM that were treated for COVID-19 at our hospital from January to April 2022 were included. We excluded three patients to whom the test-based strategy was not applied. In our test-based de-isolation strategy, RT-qPCR testing using nasopharyngeal swabs was performed twice a week in patients. We de-isolated patients when their Ct values were ≥ 30 after their symptoms resolved. The patients were categorized according to the duration needed to achieve a Ct value of >30: cluster 1 (< 20 days), cluster 2 (21-40 days), and cluster 3 (>40 days). The similarities in patients' characteristics in each cluster and the differences between the clusters were examined.
A total of 14 patients were analyzed, and the median age of the patients was 71 years old (range: 28-82 years old). Eight patients had non-Hodgkin lymphoma (NHL), four had acute leukemia (AL), and two had multiple myeloma (MM). Of the patients with NHL, four were treatment-naive or on first-line chemotherapy, while the other four had relapsed or refractory (r/r) NHL. Among r/r NHL patients, two received salvage chemotherapy, and the remaining two had opted for best supportive care. Three of the four patients with AL achieved complete remission (CR) after allogeneic hematopoietic stem cell transplantation (HSCT), while the other had a relapse after receiving salvage chemotherapy. One of the patients with MM achieved CR with maintenance therapy after autologous HSCT, while the other received sixth-line chemotherapy for refractory disease. The severity of COVID-19, which was defined by the National Institutes of Health guideline, was asymptomatic in two patients, mild in eight, moderate in one, severe in three, and critical in one. A total of 117 PCR tests were performed, and Ct values were available for 114 of them. The median time for Ct value to reach >30 was 23 days (range: 14-84 days). Five patients were categorized into cluster 1, six into cluster 2, and three into cluster 3. In cluster 1, patients were untreated or received less than three courses of chemotherapy within the past year and had mild or moderate COVID-19 infection. Patients in cluster 2 received three or more courses of chemotherapy or HSCT in the past year and had severe COVID-19 infection. Cluster 3 consisted of three patients who were treated with high-dose immunosuppressant for active graft-versus-host disease, had a history of long-term chemotherapy, and were critically ill from COVID-19 infection.
The duration of viral shedding in patients with HM was generally prolonged and stratified by treatment history and severity of COVID-19. The number of chemotherapy treatment and severity of COVID-19 may serve as useful indicators to determine whether a test-based de-isolation strategy should be followed in patients with HM.
Disclosures
Kondo:Asahi Kasei Pharmaceutica,Chugai Pharma, MSD Pharmaceutical, Dainippon Sumitomo Pharma, Otsuka Pharmaceutical: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal